About Us
Safety FAQs
These FAQs provide answers to basic questions about NBACC.
For Risk Scenarios and Safety, please click on the button below.
About Us
These FAQs provide answers to basic questions about NBACC.
For Risk Scenarios and Safety, please click on the button below.
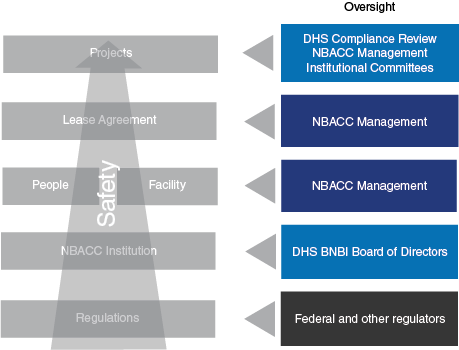
The National Biodefense Analysis and Countermeasures Center (NBACC) is a Federally Funded Research and Development Center (FFRDC) managed and operated by Battelle National Biodefense Institute, LLC (BNBI) for the Department of Homeland Security (DHS). As captured in the Strategic Plan, accomplishing the NBACC mission requires robust, special facility functions and safe, secure, compliant, and sustainable infrastructure operations. As described in the Governance section of the website, both DHS and the BNBI Board of Directors provide oversight for NBACC/BNBI. Specific oversight roles in safety, security, and compliance are summarized in Figure 1. Safety, security and compliance are an integrated part of project planning and execution, organizational structure (e.g., utilizing a tenant-landlord model for lease agreements between project and institutional staff), and oversight by regulators, DHS and the BNBI Board of Directors.
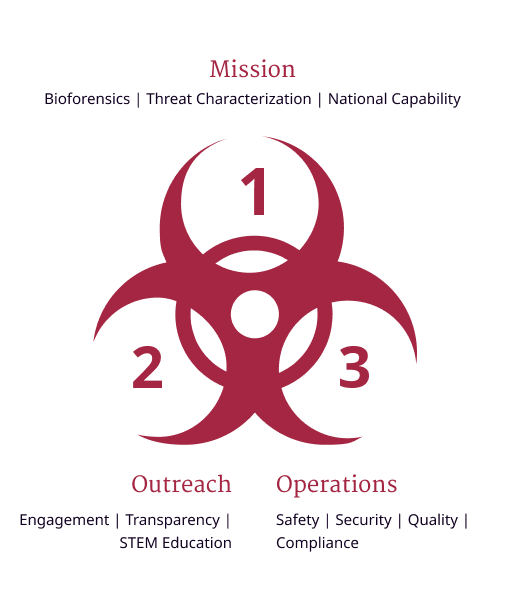
The NBACC emphasizes a culture of responsibility that begins with safety and extends expectations for rule following and human reliability (e.g., identification and remediation of error precursors) to all areas—science, operations, and outreach (Figure 2).
As shown in Tables 1, 2, and 3, compliant operations at NBACC are verified through inspections, audits, certifications and registrations. In addition, the NBACC utilizes communities of practice (e.g., professional societies, DHS S&T Laboratories, Battelle affiliates) to benchmark best practices.
NBACC External Inspections and Audits
| Agency Providing Oversight | Frequency of Inspection | Purpose of Inspection |
| Centers for Disease Control and Prevention and United States Department of Agriculture (CDC/USDA) Biosafety Level 4 (BSL-4) | Annually | Ensure compliance with Biosafety in Microbiological and Biomedical Laboratories (BMBL) and Biological Select Agents and Toxins (BSAT) regulations |
| CDC/USDA BSL-3/BSL-2 | At least every three years with unannounced inspections in-between | Ensure compliance with BMBL and BSAT regulations |
| Nuclear Regulatory Commission (NRC) | Annually | Ensure compliance with 10 Code of Federal Regulations (CFR) required for all organizations that operate a sealed source irradiator for sterilizing biological material (see http://www.nrc.gov/reading-rm/doc-collections/cfr/) |
| Department of Transportation (DOT) | Random | Ensure compliance with 49 CFR |
| Occupational Safety and Health Administration (OSHA) | Random | Ensure compliance with 29 CFR |
| Defense Security Service (DSS) | Annually | Ensure compliance with the National Industrial Security Program (NISP) |
| Fort Detrick Fire Department | Quarterly | Ensure compliance with life safety codes and fire protection standards |
| Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) | Every three years | Ensure compliance with animal welfare standards |
| The American Association for Laboratory Accreditation (A2LA) | Every two years | Ensure compliance with ISO 17025 standards for the management of quality, administrative and technical operations of tests and calibrations. ISO is an important component of bioforensics. |
| US Environmental Protection Agency (EPA) | Random | Ensure compliance with 40 CFR regulations for hazardous waste generators |
| Department of Homeland Security (DHS) Regulatory Compliance Office | Random | Ensure compliance with various regulatory requirements |
| DHS Office of Security | Annually | Ensure compliance with DHS Security Directives |
NBACC External Inspections and Programs?
| Type of Inspection | Frequency of Inspection |
| BSL-4 Inspection | Monthly |
| BSL-3 Inspection | Monthly |
| BSL-2 Inspection | Quarterly |
| General Facility Inspection | Monthly |
| Environmental Surveillance Sampling | Monthly |
| Decontamination Operations | Validated by Biological Indicators (BIs) |
| Certification of HEPA filters | Annually |
| Autoclaves | Validated with Chemical Indicators every run and BI’s quarterly. Temperature records maintained for all runs. |
| Engineering controls and building systems validated | Prior to operation of new and renovated laboratories, and annually for all laboratories |
| Biological Personnel Reliability Program | Ongoing for all employees with laboratory access |
Additional Licenses, Certifications, Registrations, and Inspections?
| Department of Justice Security Risk Assessment for all employees with access to BSAT |
| Background Investigation, applicable security clearance, and DHS Suitability for all NBACC employees |
| Maryland’s Biological Agents Registry Program Registration |
| CDC/USDA Biological Select Agent and Toxins Registration at BSL-2, 3 and 4 |
| Nuclear Regulatory Commission (NRC) License |
| Institutional Biosafety Committee (IBC) Registration with National Institutes of Health’s Office of Biotechnology Activities (NIH/OBA) |
| DHS Assessments (Risk, Vulnerability, and Threat) |
| Bureau of Alcohol, Tobacco, Firearms and Explosives (ATF) , Alcohol and Tobacco Tax and Trade Bureau Industrial Alcohol User Permit |
| Drug Enforcement Agency (DEA) Controlled Substance Registration |
| CDC Laboratory Response Network (LRN) member |
An important component of NBACC safety, security, and quality processes is the Personnel Reliability Program (PRP), which is described in the peer reviewed publication “Implementation of a personnel reliability program as a facilitator of biosafety and biosecurity culture in BSL-3 and BSL-4 laboratories” in Biosecurity and Bioterrorism: Biodefense strategy, practice, and science, vol. 11, No. 2, 2013. Significant prior approvals, as well as on-going monitoring are required (see Figure 3) in order for an employee to have laboratory access to biological select agents and toxins.
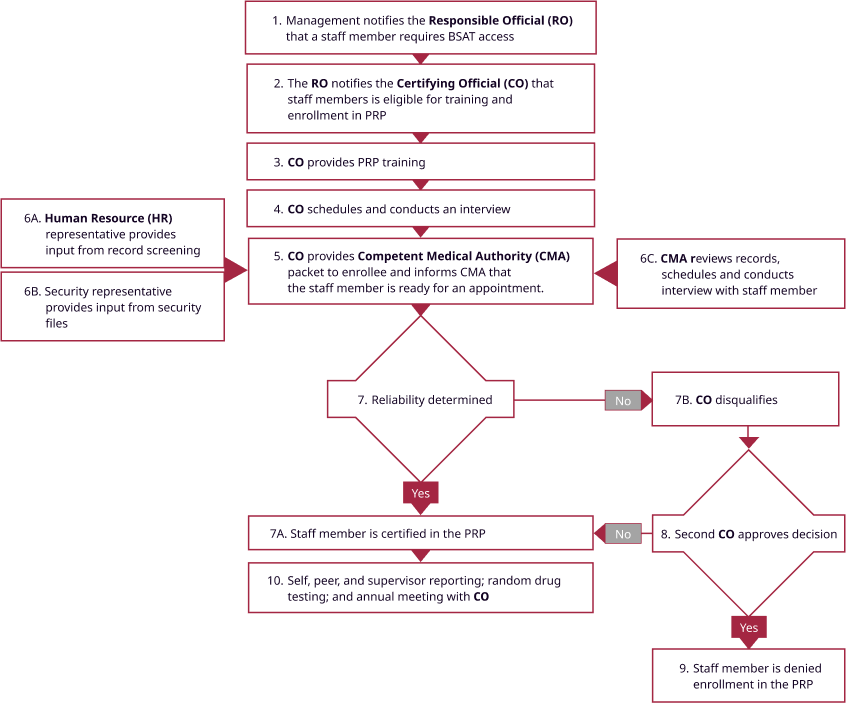
Figure 3. NBACC’s Personnel Reliability Program (PRP)
How you can get involved with us, from working with us to meeting our scientists at events.
Copyright © 2024 National Biodefense Analysis and Countermeasures Center